- Home
- /
- Capabilities
- /
- Early development and innovation
Early development and Innovation
Navigate the complex landscape of early-stage drug development
Early Development & Innovation
Our highly specialized team, composed of global experts in early development and clinical pharmacology, meticulously designs an integrated early-phase program. We possess extensive experience in conducting a variety of studies, ranging from first-in-human to drug-drug interaction, enabling informed decisions. This includes first-in-human (FIH), proof-of-concept, bioavailability/bioequivalence (BA/BE), drug-drug interaction (DDI), biosimilar, and various pharmacokinetic (PK), single ascending dose/multiple ascending dose (SAD/MAD) studies. Leveraging our advanced facilities, operational expertise, and wealth of development experience, we ensure the precise execution of your trials involving patients and/or healthy volunteers.
Our Distinct Approach:
Comprehensive Strategy
We adopt a unified strategy that encompasses every facet of the development process, enabling swifter decision-making and establishing a robust foundation for later-stage endeavors.
Cutting-Edge Facilities
Equipped with purpose-built facilities, including laboratories, we prioritize delivering the highest quality and on-time completion of your study.
Diverse Investigational Products
With extensive experience and expertise, we handle a diverse range of investigational products, including biological origin-based products, employing multiple delivery methods. This wealth of experience enables us to empower informed decisions.
Extensive expertise in conducting studies across a wide array of therapeutic areas
- Analgesics
- Anticonvulsants
- Antidepressants
- Antifungal
- Antibacterial
- Antidementia Agents
- Antimigraine Agents
- Antiretrovirals
- Antiviral
- Blood Glucose Regulators
- Blood Modifiers
- Cardiovascular Agents
- Dermatological Agents
- Gastrointestinal Agents
- Central Nervous System Agents
- Hormonal Agents
- Immunological Agents
- Ophthalmic Agents
- Phytopharmaceuticals
- Respiratory Agents
- Vaccines
- Inflammatory Bowel Diseases
Phase I Clinical Trials
At Lambda Therapeutic Research, we understand that every drug is unique. Our early development services are meticulously tailored to match your compound, your business model, and your study’s specific objectives. With a wealth of experience spanning over 25 years, our highly skilled team possesses unparalleled expertise in Phase I scientific and therapeutic domains.
Lambda has successfully completed more than 40 Phase-1 studies in the past 5 years, encompassing a diverse range of formulations, including Oral, Parenteral, Inhalers, Topical, Transdermal, Nasal Sprays, Injectables, Pessaries, Suppositories, and more.
Our CPUs:
Our state-of-the-art Clinical Pharmacological Units (CPUs) in Ahmedabad (India) and Las Vegas (USA) employ a synergistic approach across geographies, offering timeline and cost advantages. These specialized units excel in conducting early-phase studies on healthy volunteers, including special populations, across a diverse spectrum of therapeutic areas.
- One of India's largest clinical pharmacology facilities featuring state-of-the-art infrastructure and an extensive safety set-up.
- 37,000 sq ft facility
- 4 clinics with 190 beds for early-phase trials
- Dedicated 12-bed capacity for Phase-I (FIH) studies
- Dedicated Outpatient/Returns clinic
Our Broad Spectrum of Phase-I Studies Includes:
Single / Multiple Ascending Dose (SAD/MAD)
First-in-human (FIH)
Bioavailability / Bioequivalence
Drug-Drug Interaction
Food-Drug Interaction
Drug-Device Combinations
PK-PD
Proof-of-Concept
Food effect studies
Cardiac Safety studies
Inhalation studies
Dermatology studies
Human Factor studies
NORTH AMERICA
- FiH Or SAD study in Canada &US
- Faster regulatory approval
- Parallel submission for MAD study in India
- Study starts with healthy subjects followed by patient cohorts
INDIA
- Cost-effective option for subsequent Phase-1 studies
- Easier Access for Renal and Liver impaired subjects study
VALUE PROPOSITION
- Cost-effective business model (Hybrid)
- Faster Turn Around Time
- Global scientific overview
- Flexible Operational approach
Biosimilar PK/PD & Immunogenicity Studies
Lambda excels in providing comprehensive services vital for successful clinical studies supporting biosimilar marketing applications. Developing biological products is a rigorous process due to their complex protein-based molecular structure, with molecular weights ranging from 3,000 to 150,000 Daltons. This category includes Growth Hormones, Insulin, Monoclonal Antibodies (MABs), and more. Unlike chemically derived products, biosimilars require specialized assessments of Pharmacodynamic and Immunogenicity, in addition to Pharmacokinetic evaluations. Our specialized expertise is essential for the successful clinical development of these products.
Our expertise ranges from PK bioequivalence to evaluating the similarity of effects in patients. Years of analyzing drug bioequivalence and bioavailability have honed our capabilities to craft precise comparative studies, establishing biosimilarity with marketed reference drugs.
We have an impressive track record of conducting numerous studies on various biological products, including but not limited to:
Bevacizumab
Trastuzumab
Denosumab
Pertuzumab
Follicular Stimulating Hormone (FSH)
Insulin
Enoxaparin
Immunoglobulins
Bevacizumab
Trastuzumab
Denosumab
Pertuzumab
Follicular Stimulating Hormone (FSH)
Insulin
Enoxaparin
Immunoglobulins
BA/BE
With a foundation of strong scientific expertise, our Bioavailability/Bioequivalence units provide a robust platform for handling challenging studies. Globally, we offer 700+ clinical beds, including 85+ specialized beds dedicated to complex Bioequivalence (BE) studies. These units are staffed by highly trained and experienced study teams, conducting numerous studies annually. Our services encompass a wide range, including clinical conduct, monitoring, bioanalysis, and project management, aligning seamlessly with regulatory requirements. Our approach emphasizes robust recruitment and customized housing options, tailored to meet sponsor and study requirements. Impressively, we have successfully navigated over 50 inspections by leading regulatory agencies across all Lambda facilities.
- Dedicated "State-of-the-art" ICUs
- Pioneering implementation of IRIS registration
- Availability of qualified doctors round the clock
- Well-defined Standard Operating Procedures (SOPs)
- Adherence to stringent subject compliance measures
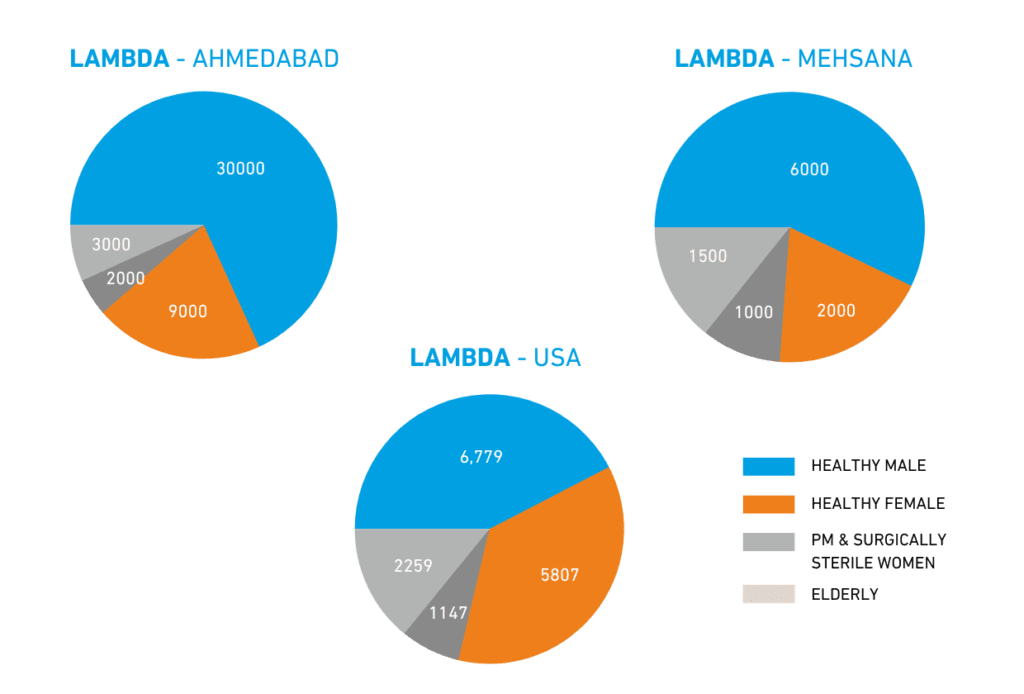
- Broad experience encompassing over 7,500 BE studies.
- Extensive database of 50,000+ healthy volunteers, including specialty populations like elderly males, non-smoker males/females, surgically sterile/post-menopausal females.
- Exclusive implementation of a complete and accurate paperless data entry system (BizNET Software), ensuring ‘On Time-Real time’ data capture from screening to the conclusion of the study.
Capability
All oral dosage forms
Injectable
Inhalations
Topical Products
Nasal Sprays
Suppositories
Transdermal
Vaginal Products
Ointments & Creams
Intravaginal Tabs
Nutraceuticals & Consumer Products Studies
In the rapidly expanding landscape of natural products and dietary supplements, clinical trials have become indispensable to ensure safety and efficacy. At Lambda, we stand by your side throughout your clinical trial journey, empowering you to thrive in the Nutraceuticals & Consumer Products market, expand your market presence, and differentiate your products. Our team of seasoned experts is dedicated to comprehending your unique requirements and providing solutions in strict accordance with regulatory standards.
Lambda has been instrumental in assisting key industry players in this segment in determining the optimal study design approach, including dosage considerations. Our expertise extends to understanding interactions between study drugs, nutraceuticals, and individuals under specific health conditions.
Our tailored solutions cater to your specific needs, offering a comprehensive range of services that span from crafting study plans to study execution, report publication, and product registration. We specialize in conducting studies related to safety and efficacy, substantiating advertisement claims, raising public awareness, and facilitating product registration across a diverse array of products.
- De-Addiction Products
- Hair Re-growth Products
- Personal Hygiene Products
- Over The Counter (OTC) Products
- Multivitamins and Nutritional supplements
- Fortified Food Products - Edible Oil, Flours, Fruit Extracts, Milk Products, Rice Extracts
Let's Unveil New Therapies Together
Connect with our experts to leverage our proven track record of clinical research excellence.