- Home
- /
- Full-service Clinical Research Capabilities
- /
- Clinical Safety & Pharmacovigilance
Clinical Safety & Pharmacovigilance
Empowering Drug Safety through end-to-end Pharmacovigilance Services
Lambda Therapeutic Research provides comprehensive drug safety & pharmacovigilance services, encompassing all clinical and post-marketing phases. Our well-established PV framework ensures thorough safety monitoring and regulatory compliance for both clinical and commercial products, offering end-to-end support across the entire life cycle of medicinal products.


Lambda Therapeutic Research provides comprehensive drug safety & pharmacovigilance services, encompassing all clinical and post-marketing phases. Our well-established PV framework ensures thorough safety monitoring and regulatory compliance for both clinical and commercial products, offering end-to-end support across the entire life cycle of medicinal products.
The LAMBDA Advantage
Experience & Expertise in Major Therapeutic Areas & Products
Expert Team:
Lambda boasts a dedicated team of over 160 professionals, comprising Physicians, Pharmacists, and PV specialists. With a vast therapeutic expertise covering over 400 active molecules, we bring a wealth of knowledge to the table.
Global Reach:
Lambda’s global reach extends across key territories, including EU, UK, North America, LATEM, APAC, and GCC & ROW countries, ensuring comprehensive Pharmacovigilance services across diverse geographic regions.
Client Base:
Lambda proudly provides end-to-end PV services to a diverse clientele of over 40 clients, ranging from large to smaller-sized pharma companies, both innovators and generic groups.
Quality Assurance and Compliance:
Our commitment to quality and compliance is demonstrated through predefined KPIs. These KPIs objectively measure the quality and compliance of all our operational deliverables, ensuring the highest standards in our services.








Core Services
- Case Processing
- Medical Information
- Drug Safety Reporting
- Risk Management Plans
- QPPV Services (UK & EU)
- Clinical Safety Management Plan
- ASIME (Analysis of Similar Events)
- PSURs, PADERs, ASRs, DSURs, PBRER & ACOS
- Literature Services
- xEVMPD Compliance
- EudraVigilance Services
- PV Consulting & Training
- Medical Writing & Publishing
- SUSAR processing & Submission
- Signal detection, evaluation & Management
- Global Compliance & Support for Regulatory Affairs
CORE SERVICES
- Case Processing
- Literature Services
- Medical Information
- xEVMPD Compliance
- Drug Safety Reporting
- EudraVigilance Services
- Risk Management Plans
- PV Consulting & Training
- QPPV Services (UK & EU)
- Medical Writing & Publishing
- Clinical Safety Management Plan
- SUSAR processing & Submission
- ASIME (Analysis of Similar Events)
- Signal detection, evaluation & Management
- PSURs, PADERs, ASRs, DSURs, PBRER & ACOS
- Global Compliance & Support for Regulatory Affairs
Operations – ‘Hub & Spoke’ Model
Our base in India serves as a pivotal hub for global back-end operations, ensuring meticulous oversight of safety processes and pharmacovigilance activities. Lambda’s ‘Hub & Spoke’ model integrates these global operations, enabling us to provide comprehensive clinical safety and pharmacovigilance solutions. We prioritize safety, ensuring the well-being of patients and the success of your trials on a global scale.


Operations – ‘Hub & Spoke’ Model
Our base in India serves as a pivotal hub for global back-end operations, ensuring meticulous oversight of safety processes and pharmacovigilance activities. Lambda’s ‘Hub & Spoke’ model integrates these global operations, enabling us to provide comprehensive clinical safety and pharmacovigilance solutions. We prioritize safety, ensuring the well-being of patients and the success of your trials on a global scale.

PV Systems
- Customized settings based on client specifications.
- Flexibility to scale up resources as per requirements.
- Management of standard and off-label customer inquiries
- Efficient management of the entire cycle from receiving inquiries to database management and response
- Response to inquiries regarding therapeutic indications, contraindications, posology, and interactions with other medicinal products
- Comprehensive knowledge of PSMF requirements across various different territories.
- Preparation and maintenance of the Summary of Pharmacovigilance System (sPhVS).
- Extensive experience in preparing PSMFs compliant with the regulations of EU, UK, Saudi Arabia, Kenya, Ukraine, India, and other health authorities.
- Dedicated and experienced team of healthcare professionals for PSMF authoring and periodic updates, working under the close guidance of the QPPV.
- Well-defined internal procedural document for the preparation of EU, UK, and other health authority PSMFs to ensure accuracy and quality.
- Management of extensive portfolio: Overseeing 100+ PSMFs annually, including new, full revisions, and updates.
- Managing 150+ SDEAs with Global affiliates of our clients
- Exchange of Adverse Drug Reactions (ADRs) with business partners through a gateway setup.
- Authoring Safety Data Exchange Agreements (SDEAs) in compliance with global regulatory requirements and reviewing existing SDEAs/PV agreements.
- Proficiency in authoring different types of SDEAs, including Marketing Authorization Holders (MAH), License partners, Distributors, Manufacturers, etc.
- QPPV services in Europe and the United Kingdom.
- Providing uninterrupted 24/7/365 coverage for health authorities.
- Comprehensive process oversight through various checks to ensure compliance.
- Continuous support and guidance to ensure PV deliverables align with guidelines
- Network of Local PV responsible persons, tailored to specific NCAs requirements.
- Dedicated QPPV and Deputy QPPV serve as a single contact point for review and deliverables.
- Team of highly qualified and extensively experienced QPPVs to establish robust PV processes.
- Guidance, training on new PV requirements, and constant monitoring via Regulatory Intelligence for major Health Authorities.
- Provide LRP/Local QPPV service in all EU countries as well as Rest of World countries (Tunisia, Morocco, Australia/New Zealand, Saudi Arabia, Kenya, Uganda, Rwanda, Namibia, Tanzania, Ethiopia, Burundi, Zimbabwe, India)
Operational Services
- Auto reads Excel sheet and converts Excel sheet into an XML format.
- Sends out valid ICSR for further processing in the case processing module (auto-import facility).
- Automates the process of triaging case activities by downloading the cases from the Canada Vigilance portal in bulk and helping them to segregate.
- Provides auto-duplicate check with the information available in the XML and helps users to decide whether the case is initial, follow-up, or duplicate.
- PvD’Code enables direct case imports, eliminating the need for labor-intensive manual data entry and significantly boosting user productivity.
- Can be used with any PV system or E2B converter and leverages an intuitive user interface to significantly reduce the manual effort required to separate the cases relevant to the MAH.
- PvD’Code offers a user-friendly interface compatible with popular web browsers such as Internet Explorer, Mozilla Firefox, Google Chrome, and Safari. Its key feature is the seamless analysis of multiple XMLs/.xlsx files received from regulatory authorities daily.
- Local literature screening as per local regulations.
- Full text procurement and translation through an approved vendor.
- Expertise in defining search strategy to screen all the potential safety articles.
- Medical literature monitoring as per EMA requirements for clients based in EU and UK.
- Weekly literature monitoring of PubMed for identification of ICSRs and Publication studies.
- Literature review process is tracked by fully validated and automated software module (LAM).
- Capability of reviewing over 500 active moieties with a turnover of over 450,000 abstract reviews annually.
- Supports MedDRA and WHO-DD browsers
- An advanced and largely automated end-to-end Web-based Pharmacovigilance (PV) solution.
- Compliant for handling a wide range of products including Vaccines, Devices, and Combination products.
- Regulatory-compliant, validated global safety database compliant with 21 CFR Part 11 and ICSR E2B R2 and R3 specifications
- Capable of seamlessly handling R2 and R3 XMLs with complete automation, significantly reducing manual intervention and ensuring accuracy.
- Equipped to generate CIOMS, MedWatch, Vaccine Vigilance forms for submission or sharing with business partners, streamlining regulatory processes
- Additional features include PvD’s Code (Partial automation), Literature automation module, and PrITR for efficient management of medical information.
- Capable of generating various line listings essential for the preparation of diverse reports, including PSUR, PADER, Signal Detection, DSUR, ASR, and more.
- Well-established process that ensures timely update to the client and its affiliates.
- Capable of handling more than 5000 entries in a year with a compliance score of >99.00%.
- Dedicated team of healthcare professionals for performing xEVMPD submission into xEVPRM database throughout the product lifecycle.
- Prepared ≥10,000 signal reports to date and currently handling ≈1500 signal reports per annum.
- Have a distinguished process to perform signal detection of Biologic products and small molecules.
- Performed by both quantitative and qualitative methods of signal detection with further validation, assessment, and prioritization.
- Performed by considering EVDAS (Eudravigilance Data Analysis System) Data, regulatory recommendation (PRAC, USFDA, etc) during signal detection activity.
- The team has experience in almost all therapeutic area products including but not limited to oncology, psychiatry, cardiovascular, immune disorders, metabolic and endocrine, etc.
- A team of healthcare professionals dedicated to ICSR case processing.
- A well-defined process for follow-ups with consumers, healthcare professionals, and reporters.
- Customized workflows for efficient management of different types of cases (e.g., Regulatory XML cases) as per client requirements.
- Capability of ICSR submissions to authorities and business partners through Gateway or Web-trader or as per country-specific requirements.
- The team has processed more than 600,000 cases to date with expertise in all therapeutic classes of drugs, including large molecules, vaccines, blood products, oncology drugs, etc.
- Well-defined procedural documentation for every aspect of case processing, including ICSR case triaging, categorization, processing, medical review and assessment, quality review, and reporting of ICSRs to different health authorities.
- Expertise across diversified therapeutic areas, including but not limited to Biosimilars, Oncology, Anti-viral, Anti-biotics, Cardiovascular Products, Anti-psychotics, etc.
- Capacity to prepare approximately 1000 Aggregate Reports (ARs) and Risk Management Plan (RMPs / REMS) per annum in line with ICH / GVP / local requirements.
- A team of expert medical writers and healthcare professionals to meet the regulatory requirements for submission of different types of Aggregate Reports (ARs) and Risk Management Plan (RMPs / REMS) worldwide, including but not limited to USA, EU, UK, Canada, Australia, Brazil, Saudi Arabia, Kuwait, UAE, Ukraine, India, etc.
- A successful track record with the highest level of quality and regulatory submission compliance during audits and regulatory inspections for different types of ARs and RMPs / REMS as below:
- Ad-hoc safety reports to meet
- Clinical Expert Statements (CES)
- Annual Summary Reports (ASRs)
- Periodic Safety Update Reports (PSURs)
- Development Safety Update Reports (DSURs)
- Periodic Benefit-Risk Evaluation Reports (PBRERs)
- Periodic Adverse Drug Experience Reports (PADERs)
- Addendum to the Clinical Overviews (ACOs / AddCOs)
- RMPs / REMS for pre-approval and post-approval regulatory requirements followed by its lifetime maintenance with any changes in risk profile or product safety information for the respective products as per the local requirement.
- Preparation of core educational materials [e.g., HCP / Patient Guide or Program or Elements to Assure Safe Use (ETASUs) and communication plans (e.g., DHPCs)] and to evaluate its effectiveness at defined intervals as per the set milestone for the different additional risk minimization measures (aRMM) and additional pharmacovigilance (APhV) activities mentioned in approved RMPs / REMS.
Support Services
- Manage training and training records efficiently using validated software – Q-Edge TMS.
- Utilize a robust learning management system for comprehensive training on global pharmacovigilance legislations, guidelines, and regulatory agency regulations.
- Offer recommendations and solutions to enhance efficiency through continuous training management, aiding in design, development, and improvement of pharmacovigilance departments.
- Establish global QMS systems and processes aligned with ICH guidance and in compliance with local regulations.
- Utilize validated software – Q-Edge QMS for streamlined management of procedural documents, change controls, deviations, and CAPA management.
- Implement a comprehensive QMS covering organizational structure, responsibilities, procedures (SOPs, work instructions, and guides), resource management, compliance, and record management.
- Conduct monthly monitoring of key performance indicators
- Track, analyze, and implement appropriate CAPA plans to prevent recurrence of errors.
- Global Compliance and Quality Assurance Meetings (GCQA) for complete oversight of PV performance indicators and to discuss improvement plans as needed.
- Expertise in vendor qualification and evaluation processes to assess vendor capabilities.
- Provide support in conducting audits of MAH business partners, MAH affiliates, and service providers of MAH.
- Robust process encompassing planning, risk assessment, audit conduct, reporting, CAPA tracking, and closure for audits of internal key PV processes and contracted vendors.
- Ensure audit and inspection readiness, focusing on appropriate CAPA planning with comprehensive investigation, root cause analysis, further assessment, and defined timeline for CAPA implementation.
- Extensive global experience in managing regulatory inspections, including EMEA (EMA, MHRA, HPRA, ANSM, SUKL, AIFA, FAMHP, Dutch Inspectorate, State Agency of Medicines, SFDA), North America (US FDA, Health Canada), and Rest of the World (TGA Australia, CDSCO India, Ministry of Health Kazakhstan).
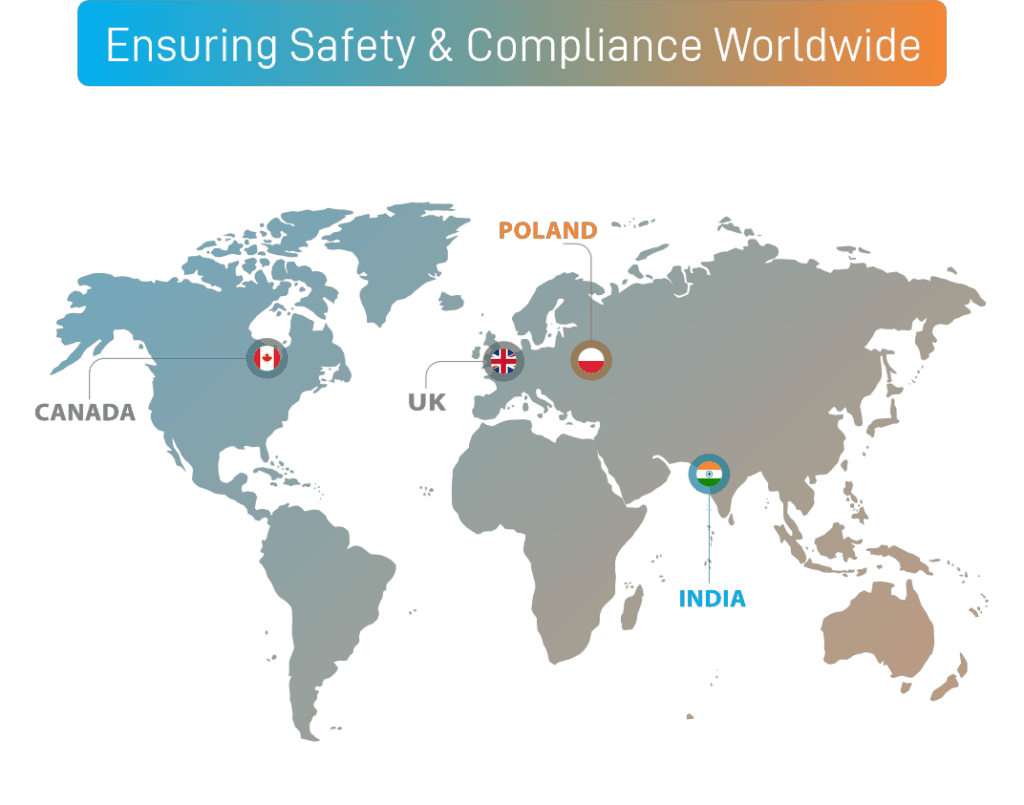

UK HQ
(UK QPPV & MI Services)
POLAND
(EU QPPV, LRP & MI Services)
INDIA
(PV Delivery & MI Services)
CANADA
(MI Services)
Let's Unveil New Therapies Together
Connect with our experts to leverage our proven track record of clinical research excellence.












