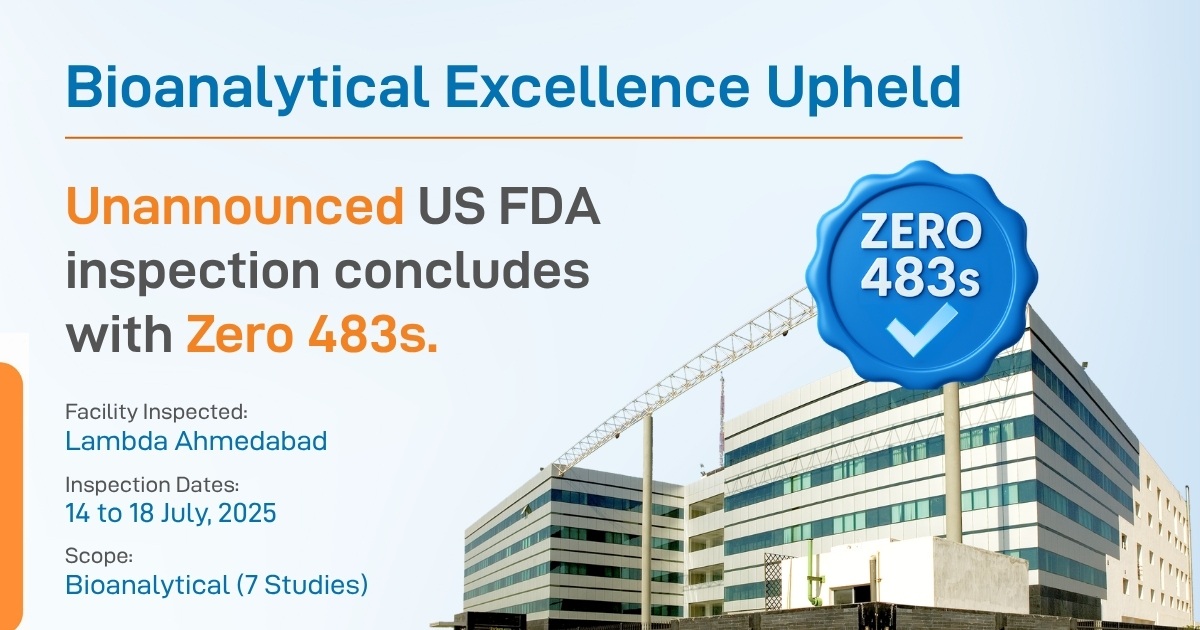
From Compliance to Confidence – Lambda Pharmacovigilance Milestones 2025
Lambda Therapeutic Research provides QPPV services across the EU and UK, offering companies a reliable framework to meet regulatory expectations and maintain inspection readiness at all times.