This case study explores advanced techniques for peptide analysis, focusing on quantifying Desmopressin in plasma using LC-MS/MS. It highlights innovative strategies to achieve ultra-low limits of quantification, enhancing assay sensitivity and reproducibility for complex biological matrices.
Quantifying small peptide molecules like Desmopressin in complex biological matrices is a significant challenge in analytical chemistry. Desmopressin, a synthetic analogue of the human hormone arginine vasopressin, is used to treat conditions such as diabetes insipidus and nocturnal enuresis. However, accurately quantifying Desmopressin, particularly in plasma samples, is difficult due to its low concentration levels and the presence of interfering endogenous materials. To overcome these challenges, a highly sensitive, robust, and reliable assay is essential for its accurate quantification.
A recent study aimed at developing such an assay for Desmopressin oral lyophilizate tablets (240 mcg) took a novel approach to improve liquid chromatography-tandem mass spectrometry (LC-MS/MS) quantitation. The objective was to achieve an ultra-low limit of quantification (LLOQ), targeting levels as low as 1 pg/mL. The solution involved optimizing various methodologies, including the summation of similar product ions, to enhance the assay’s performance. This blog explores how this innovative concept evolved, its application, and the resulting improvements in sensitivity and reproducibility.
Methodology Development: Approaches to Achieving LLOQ
Several key strategies were employed to achieve the desired LLOQ for Desmopressin quantification: Outcome of different methodologies adopted is tabulated below,
| Methodology | Trials Done | Outcome |
|---|---|---|
| Using Highly Sensitive LC-MS/MS Instruments | All available highly sensitive LC-MS/MS instruments were evaluated | LLOQ achieved close to 10 pg/mL |
| Optimization of Mass Spectrometry | All mass spec parameters (source and compound depended parameters) were optimized to enhance sensitivity | LLOQ achieved close to 10 pg/mL |
| Optimization of chromatographic conditions / sample extraction methods | Different type of Columns (including different dimensions and chemistries), organic solvents, buffers, chromatographic conditions were evaluated to optimize sensitivity. Different extraction methods like SPE, LLE, SPE+PPT. and others were evaluated. | LLOQ achieved close to 10 pg/mL |
| Using Large Matrix Volume for Analysis | Considering 3 period study with 18 time points/period, maximum aliquot volume feasible was 0.75mL | LLOQ achieved close to 10 pg/mL using this aliquot volume |
| Adduct Ion Evaluation/Summation of different product Ions | Both concepts were evaluated to enhance sensitivity | Adduct ion not feasible for this molecule. Chromatographic baseline was significantly higher in other product ion thereby making quantification difficult using summation approach. |
| Derivatization | Different derivatization solution/approaches were evaluated to enhance the sensitivity | No reported literature available on derivatization of Desmopressin. Molecule structure has no such positions for effective derivatization |
Results
- Using various optimized methodologies, the lowest LLOQ achieved was close to 10 pg/mL.
- The summation of identical product ions approach was discussed internally and successfully applied to the Desmopressin method.
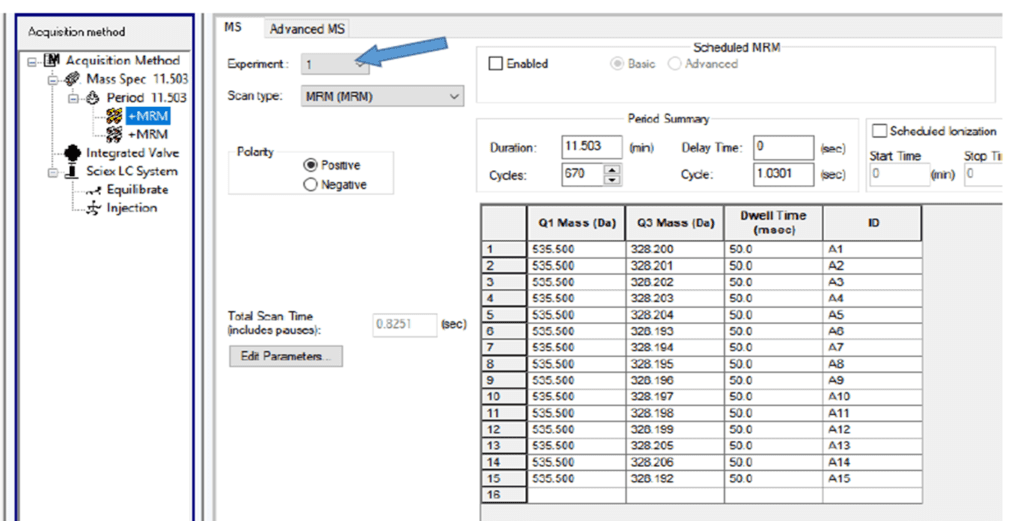
- The MRM was prepared with a minor modification in the product ion, keeping the parent ion consistent across different MRMs.
- Data were evaluated using 3, 5, 7, 10, and 15 identical product ion summations.
- Improved sensitivity, selectivity, reproducibility, and signal-to-noise (S/N) greater than 5 were achieved with the summation of 15 product ions.
- The entire method development and validation were performed using this approach and subsequently applied to study sample analysis.
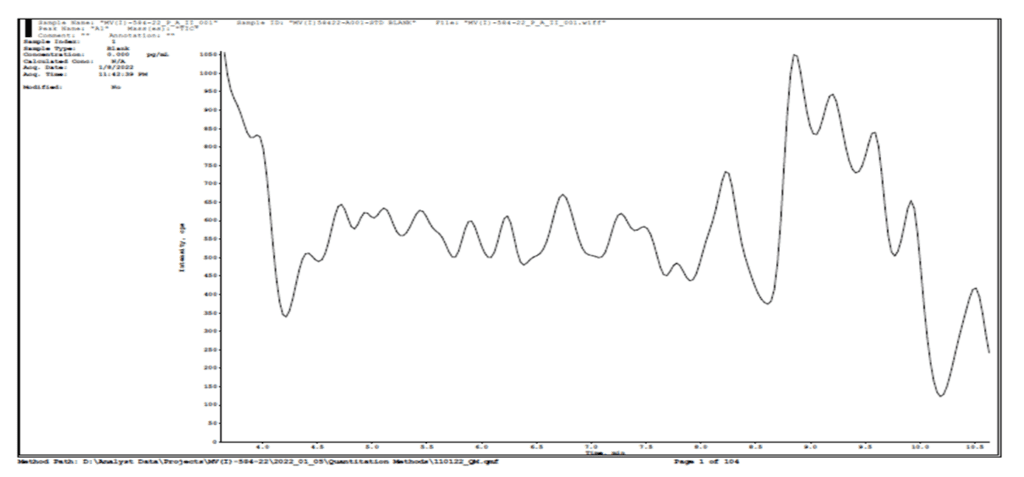
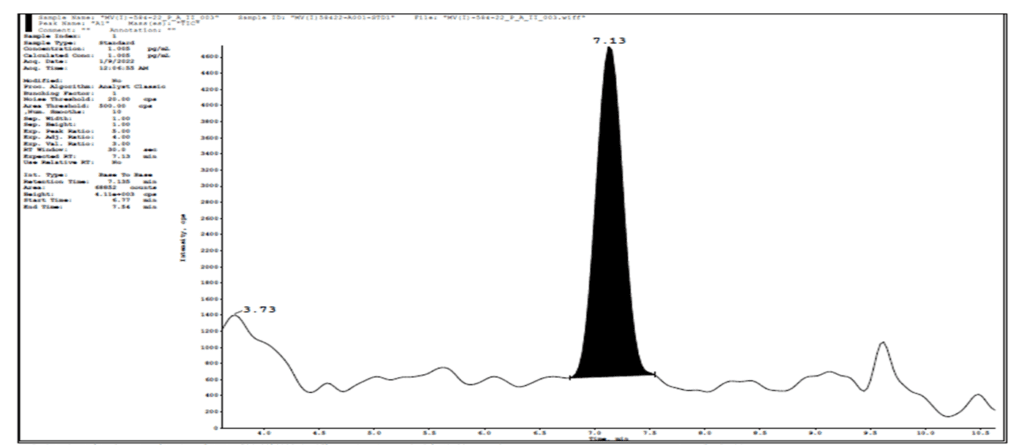
Global statistics of Three precision and accuracy run
| DQC(1/5) | HQC | MQC | LMQC | LQC | LOQQC | |
|---|---|---|---|---|---|---|
| Mean (pg/mL) | 312.5132 | 83.3915 | 34.8341 | 9.1523 | 2.9682 | 1.1194 |
| SD± | 12.27141 | 2.45568 | 1.60563 | 0.39670 | 0.15825 | 0.10517 |
| Precision (%CV) | 3.9 | 2.9 | 4.6 | 4.3 | 5.3 | 9.4 |
| Nominal value (pg/mL) | 299.322 | 78.998 | 32.389 | 8.421 | 2.947 | 1.032 |
| Accuracy % | 104.1 | 105.6 | 107.5 | 108.7 | 100.7 | 108.5 |
| n | 18 | 18 | 18 | 18 | 18 | 18 |
The entire method development and validation were performed using this approach and subsequently applied to study sample analysis.
Successful Concept Application
The summation approach was applied to five separate Desmopressin studies, all of which concluded successfully. A key performance indicator (ISR) data was as follows:
Total ISR Samples Selected:
676
ISR Samples Meeting Criteria:
603
Percentage of Samples meeting Criteria:
89.2%
These results demonstrate the effectiveness of the summation approach in ensuring reliable and reproducible quantification of Desmopressin across multiple studies.
Benefits of the Product Ion Summation Concept
The summation of similar product ions concept offers several key benefits:
- Low pg/mL Quantification: Enables quantification of molecules at extremely low levels, making it suitable for peptide analysis in biological matrices.
- Medium-Sensitivity Instrument Use: Allows for the use of medium-sensitive instruments instead of highly sensitive ones, making the analysis more accessible and cost-effective.
- Multiple Assays in a Single Study: The approach allows for the analysis of multiple assays within the same study, effectively avoiding aliquot volume constraints.
- Ethical Benefits: Reduces the volume of blood required from study participants, minimizing ethical concerns related to blood loss.
- Replicate Design Flexibility: The method supports fully replicated study designs, further enhancing the robustness of results.
Conclusion
The results generated using the summation of similar product ions concept are precise, accurate, and reproducible. This concept can also be applied to other method developments and sample analysis of different molecules.
Extrapolation of Summation Concept
Initially, the acquisition method was prepared by keeping 15 different MRMs, and quantification was performed accordingly. In some software platforms, the same purpose was achieved by summing MRMs in the quantitation file instead of the acquisition file. This approach helps avoid excessive dwell time-related issues.
Currently, two approaches are in place based on software limitations:
- A single MRM in the acquisition file, followed by summation of MRMs in the quantitation file.
- Multiple MRMs in the acquisition file, with the same MRMs used for quantitation of analytes.
Both concepts have been evaluated and tested across various methods. Around 7-8 pivotal studies using different methods have been submitted to regulatory agencies, and no queries have been raised on this concept to date.
Below is summarized information for methods developed and validated using this concept, along with the reasons for its adoption. Several other methods are ongoing using this concept.
| Molecule Name | LLOQ | Summations | Purpose |
|---|---|---|---|
| Ezetimibe | 0.075 ng/mL | 02 | Lower aliquot volume available for analysis |
| Digoxin | 25 pg/mL | 03 | 4 different methods within the same study |
| 15-Hydroxy Lubiprostone | 1 pg/mL | 02 for ISTD only | Helped in avoiding cross talk |
| Ivermectin B1b | 5 pg/mL | 02 | Lower aliquot volume available for analysis |
About Lambda
Lambda & Novum delivers full-service CRO services to the innovator, biotech, and generic pharmaceutical industries worldwide. With a strong global presence in India, the USA, Canada, the UK, Spain and Poland, we bring specialized expertise to every project. Our focus on secure IT infrastructure and automation ensures timely project delivery and strict adherence to international regulatory standards. Lambda’s exemplary regulatory track record includes over 60 successful inspections and audits by esteemed authorities, including the USFDA, EMEA, MHRA, EU member states, and other global regulatory bodies, in the past five years.
Lambda’s commitment to excellence has garnered significant recognition, including the ‘Best Indian CRO’ award from Frost & Sullivan (USA) and the ‘Great Indian Workplace’ title from UBS Transformance. Recent accolades include the ‘Regulatory Excellence’ Award at the CPhI Pharma Awards 2023 and the ‘Industry Partner of the Year’ Award at the Global Generics & Biosimilar Awards 2023.
Lambda offers a wide range of bioanalytical capabilities specializing in cell-based assays, biomarkers, immunogenicity, and pharmacokinetics (PK). With state-of-the-art facilities and a team of experienced scientists, Lambda provides comprehensive solutions for the analysis of small molecules, biomarkers, biologics, and therapeutic trace elements.




