- Home
- /
- Therapeutic Expertise
- /
- Biosimilar Development
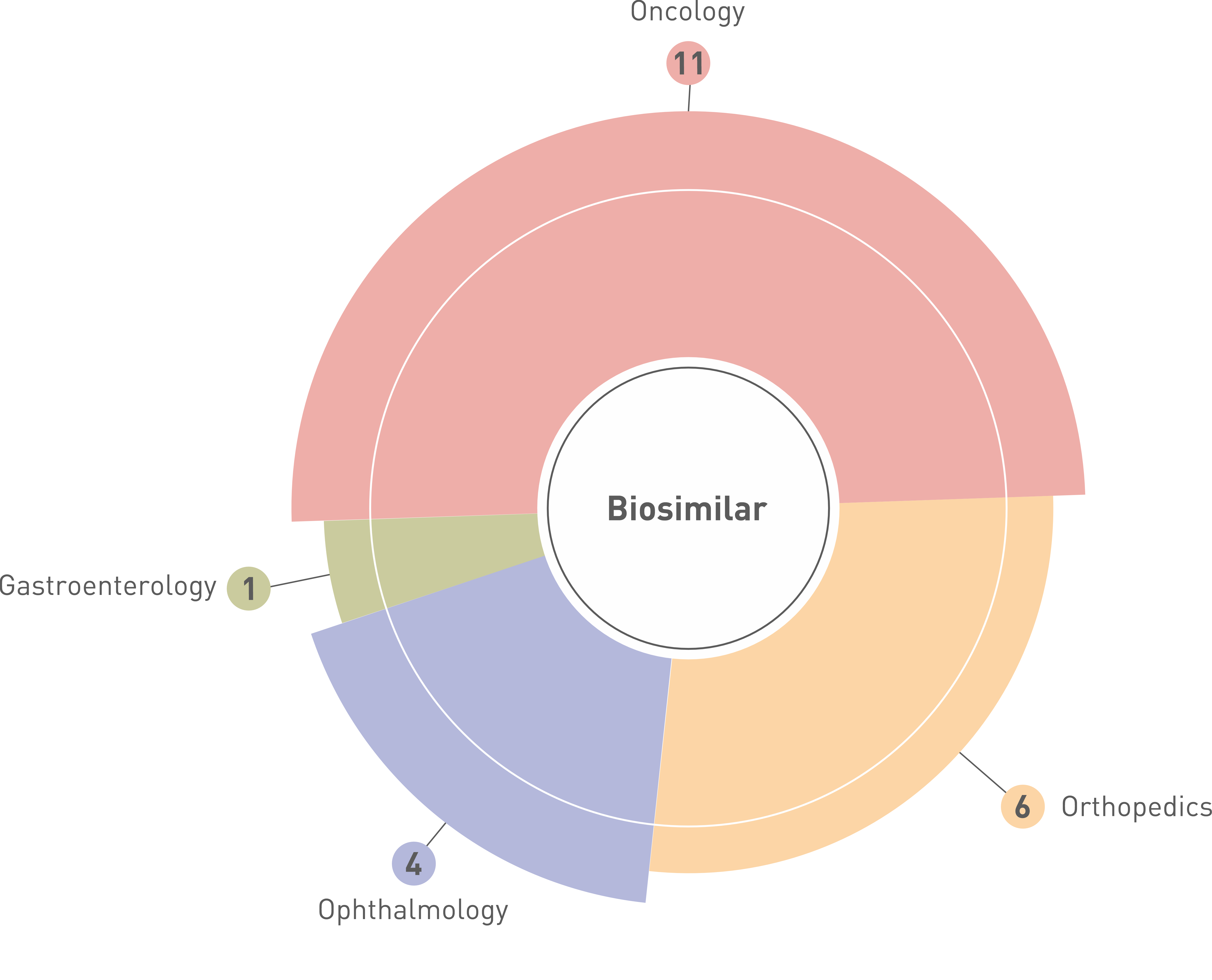
Biosimilar Development
In a world where access to life-saving biologics is often limited, the demand for cost-effective biosimilar solutions is more critical than ever. With 25 years of legacy as a trusted CRO, we understand the complexities of bringing these vital products to market and have a proven track record in biosimilar development. Our expertise includes designing effective development plans and regulatory strategies for your biosimilars, ensuring successful program execution.
Lambda has extensive experience in conducting clinical trials for biosimilars across multiple therapeutic areas, ensuring regulatory compliance and high-quality data generation. Our expertise includes pharmacokinetic (PK), pharmacodynamic (PD), immunogenicity, and efficacy studies for a range of biosimilars, including, Vedolizumab, Bevacizumab, Denosumab, Pertuzumab, Rituximab, Trastuzumab, Trastuzumab Emtansine, Aflibercept, Ranibizumab, and Teriparatide.
Indication Experience for Biosimilar Development:
- Metastatic Breast Cancer
- Non-Hodgkin’s Lymphoma
- Multiple Cancer Indications
- Solid tumours with bone metastases
- Bone Metastases from Advanced Malignancies
- Metastatic Breast Cancer or metastatic gastric cancer
- Metastatic Non-Squamous Non-Small Cell Lung Cancer
- Locally Advanced, Metastatic Non-Squamous Non-Small Cell Lung Cancer
- Locally Advanced, Recurrent or Metastatic Non-Squamous Non-Small Cell Lung Cancer
- Retinopathy of Prematurity
- Age related Macular Degeneration
- Neovascular (Wet) Age-Related Macular Degeneration (AMD)
- Osteoporosis
- Postmenopausal Osteoporosis
- Standard of care in fracture healing
- Postmenopausal Women with Osteoporosis
- Postmenopausal Women and Men with osteoporosis
- Ulcerative Colitis
We navigate global market requirements and operational challenges, making us your ideal partner for expediting biosimilar development and increasing accessibility. With a proven track record in biosimilar development, Lambda supports sponsors in navigating the complexities of regulatory submissions and clinical trials, delivering high-quality solutions for global markets.
Partner with Lambda Therapeutic Research & Novum Pharmaceutical Research Services, leveraging years of expertise to accelerate your clinical development programs.
Our Comprehensive Capabilities:
- Biostatistics
- Bioanalytical Services
- Clinical Development Plans
- Regulatory Affairs
- Clinical Supplies Management
- Pharmacokinetic and Pharmacodynamic Support
Our Presence
We are aggressively expanding globally and are confident in our research initiatives, propelling us toward a trajectory of excellence.

Corporate Office – Ahmedabad
Lambda House, Plot No. 38,
Survey No. 388, Near Silver Oak University,
S.G. Highway, Gota, Ahmedabad - 382481,
Gujarat, India.
Representative Office
Poland (Warsaw)
Representative Office
UK (London)
Representative Office
Canada (Toronto)
Representative Office
US (Las Vegas)
Representative Office
Spain (Barcelona)
Let's Unveil New Therapies Together
Connect with our experts to leverage our proven track record of clinical research excellence.