This blog explores a novel LC-MS/MS method for quantifying Free DM1 in human plasma to support clinical studies of trastuzumab emtansine (ADC). It covers the scientific rationale, key bioanalytical challenges, optimized methodology, and validation results demonstrating precision, accuracy, and stability for reliable pharmacokinetic assessment.
Novel Apsects
This research introduces a novel LC-MS/MS method for quantifying Free DM1 in human plasma, specifically tailored to support clinical studies of trastuzumab emtansine, an antibody drug conjugate (ADC formulation). The methodology addresses challenges related to the under- or overestimation of Free DM1, which is crucial for accurately characterizing the pharmacokinetics of Free DM1 from clinical samples.
Understanding Trastuzumab Emtansine and the Need for Free DM1 Quantification
Trastuzumab emtansine (T-DM1) is an ADC comprising the humanized monoclonal antibody trastuzumab conjugated to the highly potent cytotoxic agent DM1. Trastuzumab is covalently linked to DM1 (a maytansine derivative) via the stable thioether linker MCC (4-[N-maleimidomethyl]cyclohexane-1-carboxylate). DM1 is attached to lysine residues on the antibody using the heterobifunctional reagent SMCC (trans-succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate). The term “emtansine” refers to the MCC-DM1 complex.
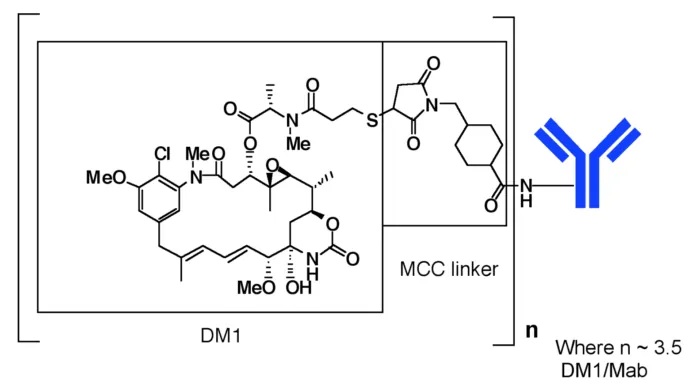
Evaluating the pharmacokinetics of T-DM1 and its components (trastuzumab and DM1) enables comprehensive profiling of their distribution and elimination phases. This is essential for demonstrating descriptive comparability between biosimilar products and reference drugs in terms of absorption, bioavailability, distribution, and elimination. This blog discusses the bioanalytical challenges in determining Free DM1.
DM1, used as chemotherapy, can cause side effects. To mitigate these, researchers developed ADCs where DM1 is bound to trastuzumab via linkers. Identifying the free form of DM1 in clinical samples is critical, and a robust bioanalytical method plays a key role.
Since DM1 contains a free sulfhydryl group, it may dimerize or react with other thiol-containing molecules in plasma upon release from T-DM1. This makes bioanalytical quantification challenging, as underestimation can occur. Additionally, the low levels of Free DM1 and significant intra- and inter-day variability impact method precision and accuracy. To address these issues, our research presents a novel LC-MS/MS method designed to overcome these challenges and provide reliable quantification of Free DM1 in human plasma, especially in ADC studies.
Method
The method involves several steps: reduction, alkylation, protein precipitation, and solid-phase extraction. Key parameters such as sample volume, reagent strength and volume, and incubation time were optimized to ensure consistent and reproducible reactions. The alkylated sample is quenched with an acidic alcoholic solution, processed via solid-phase extraction, and analyzed using chromatography on a C18 column. The method was validated for:
- Linearity
- Stability
- Carry-over effect
- Re-injection reproducibility
- Precision
- Accuracy
- Selectivity
Quality control (QC) samples were prepared using working standards, DM1 dimer, and formulation standards. The method demonstrated excellent precision and accuracy across all concentration levels.
Key Optimization Steps:
- Reducing reagent: TCEP was selected to break disulfide bonds.
- Alkylating reagent: Since disulfide bond formation is a reversible reaction, it is important to block free thiol groups to prevent reformation. N-ethylmaleimide (NEM) was used to alkylate the thiol groups, thereby preventing the reformation of disulfide bonds.
- Incubation: Optimized incubation period followed by quenching samples with acidic alcoholic solvent.
- Desalting: Samples were cleaned using cartridges and eluted samples were injected for analysis..
- Quality Control Sample Preparation and Monitoring: Quality control samples were prepared using working standards along with formulation samples and DM1-Dimer standards. As these QCs were allowed to monitor this complex process followed for study sample analysis, it enables the monitoring of the accuracy of the reported data for study samples.
The concept and methodology used to validate this assay make it unique, precise, and accurate for its intended purpose.
Result
The validated method shows excellent linearity, stability, and precision. Global statistics from formulation QC runs confirm its reliability. Matrix stability experiments, including bench-top and freeze-thaw stability, further validate the robustness of the approach.
Precision and Accuracy:
Working Standard QCs:
- Within-run: Precision: 1.0% to 11.1%; Accuracy: 92.9% to 107.0%
- Between-run: Precision: 3.7% to 7.9%; Accuracy: 96.5% to 104.5%
DM1 Dimer QCs:
- Within-run: Precision: 2.3% to 8.3%; Accuracy: 93.2% to 110.3%
- Between-run: Precision: 4.4% to 8.2%; Accuracy: 97.4% to 102.7%
Formulation QCs:
- Within-run: Precision: 3.4% to 9.1%; Accuracy: 98.1% to 103.7%
- Between-run: Precision: 2.0% to 6.7%; Accuracy: 88.8% to 107.6%
Matrix Stability:
- Bench-top stability: Established for 17 hours.
- Freeze–thaw stability: Verified across 5 cycles.
- Long-term stability: Confirmed for 224 days at −65 ± 10 °C in a freezer.
Incurred Sample Reproducibility:
Over 95% of samples met acceptable criteria, confirming the method’s reproducibility for incurred samples.
Conclusion
This validated LC-MS/MS method offers a selective, precise, accurate, and highly sensitive approach for quantifying Free DM1 in human plasma. It plays a critical role in supporting clinical studies of ADC molecules, ensuring reliable pharmacokinetic assessments through robust bioanalytical performance.
About Lambda
Lambda & Novum delivers full-service CRO services to the innovator, biotech, and generic pharmaceutical industries worldwide. With a strong global presence in India, the USA, Canada, the UK, Spain and Poland, we bring specialized expertise to every project. Our focus on secure IT infrastructure and automation ensures timely project delivery and strict adherence to international regulatory standards. Lambda’s exemplary regulatory track record includes over 60 successful inspections and audits by esteemed authorities, including the USFDA, EMEA, MHRA, EU member states, and other global regulatory bodies, in the past five years.
Lambda offers a wide range of bioanalytical capabilities specializing in cell-based assays, biomarkers, immunogenicity, and pharmacokinetics (PK). With state-of-the-art facilities and a team of experienced scientists, Lambda provides comprehensive solutions for the analysis of small molecules, biomarkers, biologics, and therapeutic trace elements.



